Do you think getting older happens "slowly"? Learn all about aging biomarkers in humans and non-human primates—explained in one comprehensive article!
Release time:
2025.09.28
“Time may not speak, but it leaves its fingerprints—at the molecular level.” Why do some people still brimming with energy at age 60, while others already feel drained and overwhelmed? The answer lies hidden within biomarkers of ageing. On September 11, Nature Reviews Molecular Cell Biology published a comprehensive review titled “Biomarkers of ageing in humans and non-human primates,” which, for the first time, systematically maps out the “fingerprint landscape of ageing” across multiple levels—from cells and organs to the entire organism—centering specifically on primates as the main focus.
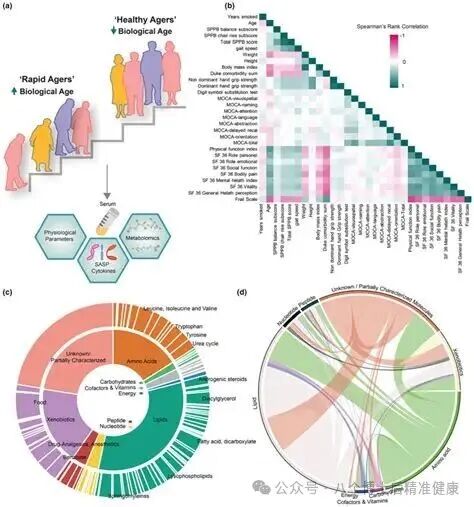
More importantly, these biomarkers are not just "descriptions"—they also serve as the "measuring tools" for future interventions and assessments. Building on this review as its framework, and drawing from authoritative research conducted in recent years, we present you with a comprehensive, verifiable, practical, and actionable "Panoramic Guide to Aging Biomarkers"! (Nature)
1. NEWS
Cellular Level: The Common Language of Ten Types of "Aging Fingerprints"
Point the microscope at the cells, and aging reveals itself through ten common "fingerprints": ① Cell cycle arrest (triggered by the p53–p21 and p16–Rb pathways), accompanied by decreased levels of Ki-67/PCNA and reduced BrdU/EdU incorporation—these are clear signals that "the brakes have been applied"; ② Loss of genomic integrity, marked by increased γ-H2AX/53BP1 damage foci, telomere-associated lesions (TAF/TIF), and elevated 8-oxoG/8-oxodG levels, indicating a decline in DNA repair capacity; ③ Telomere shortening resonates in sync with replicative senescence—a phenomenon repeatedly confirmed across human and various non-human primate fibroblasts; ④ Epigenetic instability: global depletion of H3K9me3, loosening of heterochromatin, and derepression of ERV/LINE-1 elements.
⑤Nuclear structural abnormalities: loss of lamin B1/lamin B2/LAP2, disruption of nuclear pore and nucleolar homeostasis; ⑥Mitochondrial dysfunction: elevated ROS/4-HNE/mtDNA mutations, reduced mitochondrial membrane potential and ATP levels, and imbalanced mitochondrial autophagy; ⑦Lysosomal dysfunction: accumulation of lipofuscin and increased SA-β-gal activity; ⑧Protein homeostasis breakdown: impaired ribosome biogenesis and translation regulation, along with the misfolding and aggregation of proteins (including β-amyloid, tau, and PKM2 aggregates); ⑨Metabolic dysregulation: hyperactivation of mTOR, lipid droplet deposition, ELOVL2 methylation, and altered NAD⁺/NADH ratio; ⑩SASP: secretion of inflammatory factors such as IL-1β, IL-6, IL-8, MMPs, GDF15, and amplification of inflammation via retrovirus-like particles. Combining multiple indicators is the gold standard for identifying "senescent cells," offering greater robustness compared to using a single marker! (Nature)
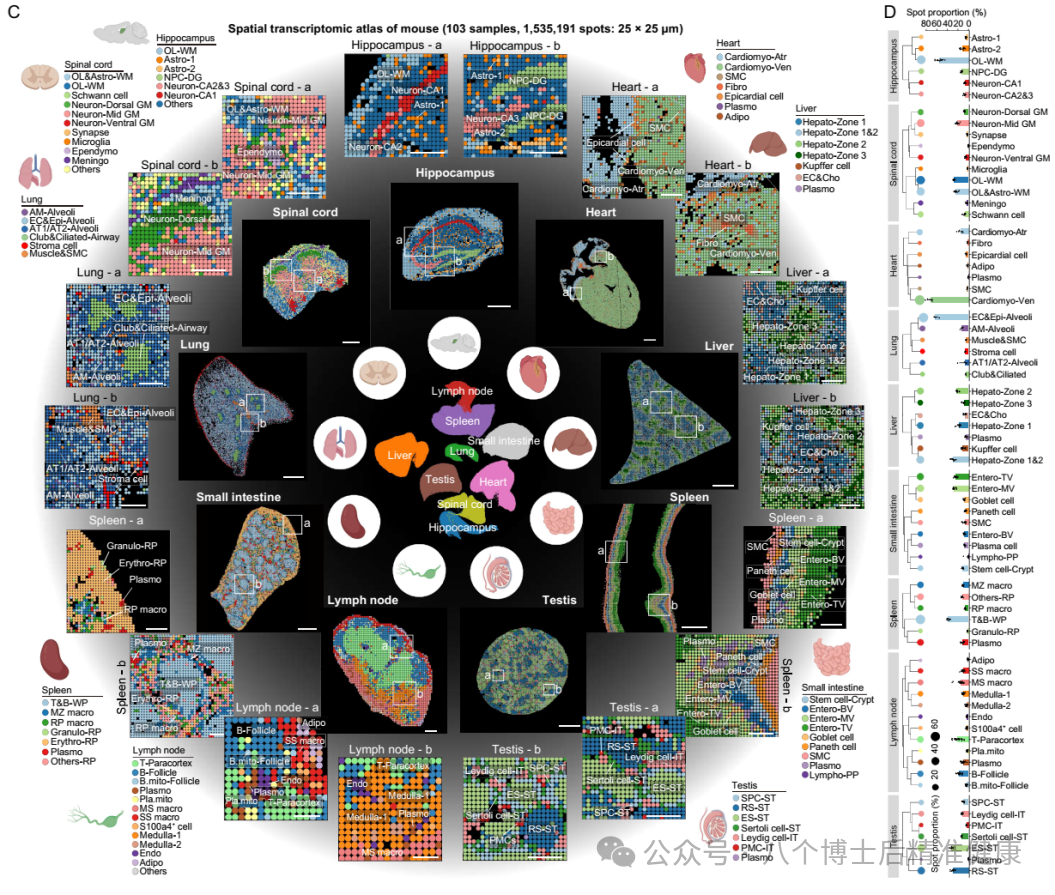
Advanced tip: DNA methylation isn’t about “less being older”—what truly matters is the epigenetic clock trained specifically on particular CpG sites (detailed later); meanwhile, ERV/LINE-1 repression relief can trigger “inflammaging” via the cGAS–STING pathway, serving as a critical bridge linking cellular and systemic levels. (Nature)
Section 2 NEWS
Organization and Organ Systems: From Skin to Brain, Why Aging "Each in Its Own Beautiful Way"
Skin: The junction between the true epidermis becomes flatter, while collagen density in the dermis decreases. Downregulation of KRT14, KRT15, and COL17A1, combined with the DNA methylation clock, collectively suggests an upward shift in "biological age."
Central Nervous System: Brain atrophy, along with declining white matter integrity and impaired cognitive-motor functions, progresses in parallel. Elevated levels of circulating factors such as NfL, GDF15, and BCAN can serve as "remote biomarkers," while increased CHIT1 in the spinal cord emerges as a new signaling indicator.
Cardiovascular System: Valve and vessel calcification intricately intertwine with arterial stiffness and endothelial dysfunction; elevated circulating cTnT/BNP levels are linked to enhanced local ACE/Ang II expression, reflecting an aging-related cardiovascular phenotype. A recent multi-tissue proteomic atlas suggests that proteins such as SAA1/2, SAP, and IGHa may emerge as novel biomarkers—highlighting that "arterial aging first" could serve as a critical "hub" in systemic aging processes.
Lungs: Thickening of bronchial walls, enlarged alveoli, increased fibrosis and fat deposition; upregulation of ACE2 may explain the molecular basis for age-related susceptibility to respiratory viruses.
Liver: Thickening of sinusoidal endothelium and reduced fenestrations, accompanied by decreased liver volume and blood flow; upregulation of ACSL4, SCAD, CYP1A2, and PCSK9, coupled with SREBP2 activation, collectively contribute to lipid metabolism abnormalities. Meanwhile, elevated serum alkaline phosphatase/γ-GT levels and increasing cholesterol/triglyceride ratios with age can serve as useful peripheral indicators.
The immune system: Thymic involution, a decline in memory B cells/dendritic cells, and an increase in circulating monocytes and memory T cells with aging; upregulation of phenotypes such as CD28/GZMK/NKG2C/PD-1, along with non-coding RNAs NEAT1/MALAT1, collectively characterize "immunosenescence."
Adipose tissue: Subcutaneous fat decreases, while visceral fat increases. Epicardial fat shows age-related elevation in IgG levels and is negatively correlated with PPARγ/leptin; adiponectin/leptin and other factors can serve as circulating biomarkers.
Skeletal muscle–bone–joint: Muscle fiber atrophy accompanied by fatty infiltration, decreased bone density, and cartilage matrix remodeling; p16/p21/lamin B1 marks senescent cells, while elevated transcriptional noise persists across multiple tissues.
Kidneys: Decreased nephron number, thinning of the renal cortex, and reduced podocyte density; downregulation of WT1/podocin, along with increased expression of fibrosis markers. Clinical indicators such as eGFR, urinary albumin, and creatinine levels align with these molecular signals. (Nature)
Three NEWS
Individual Level: Universal Metrics and the "Ageing Clock"
Common general indicators—such as physical fitness and cognitive functions (e.g., walking speed, grip strength, memory)—are more sensitive to "healthy lifespan." Meanwhile, serum inflammatory markers like IL-6, CRP, and S100A8, along with the neurodamage marker NfL, tend to rise with age, while proteins like Klotho, total protein, uridine, and taurine show a downward trend. Additionally, elevated levels of 8-oxoG in urine and cerebrospinal fluid provide a stable "out-of-system sampling" window, spanning both humans and rhesus monkeys. (Nature)
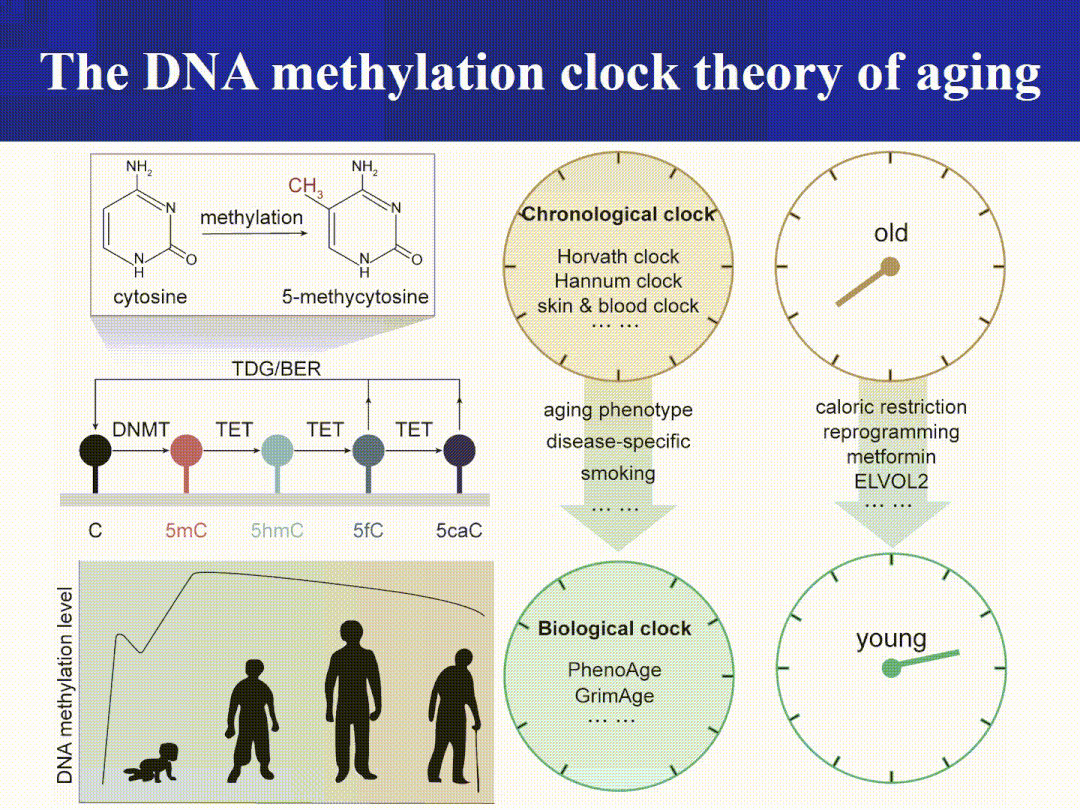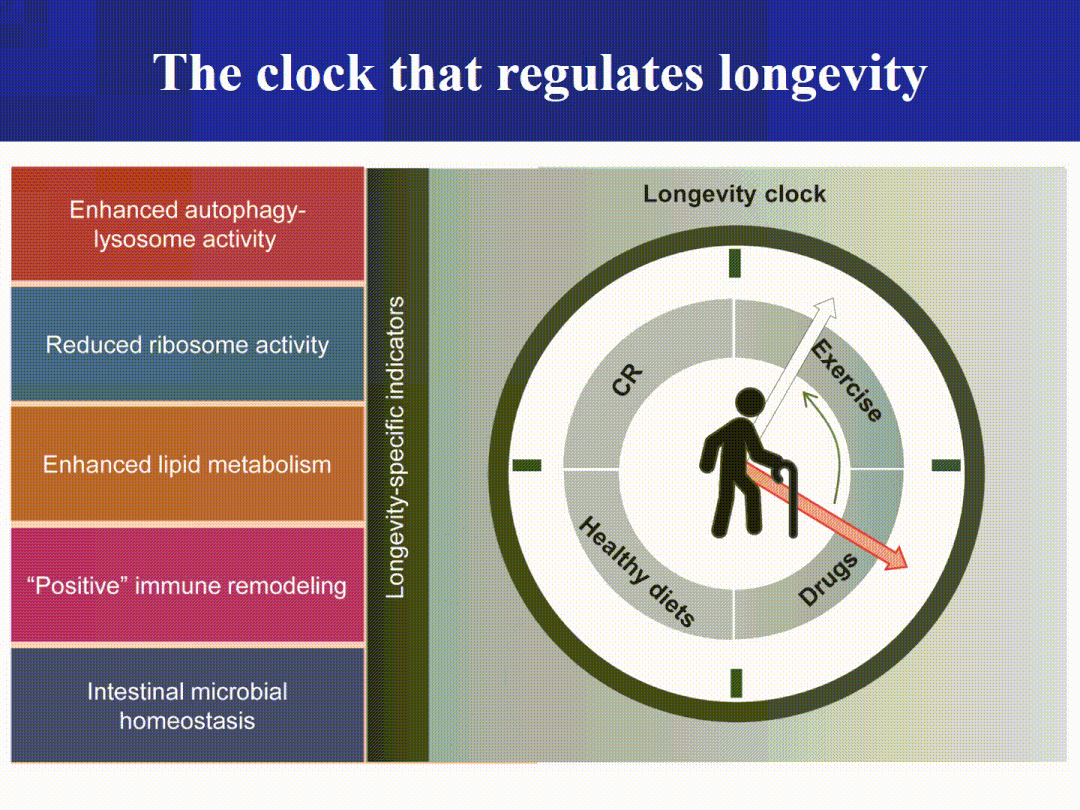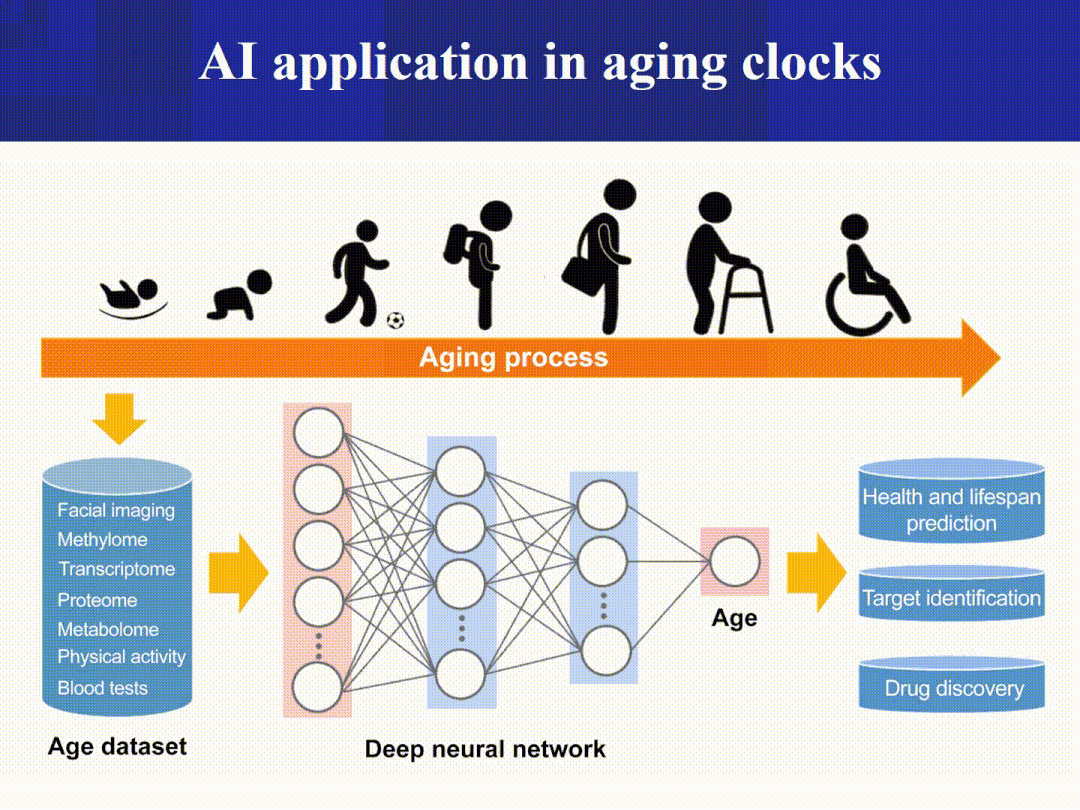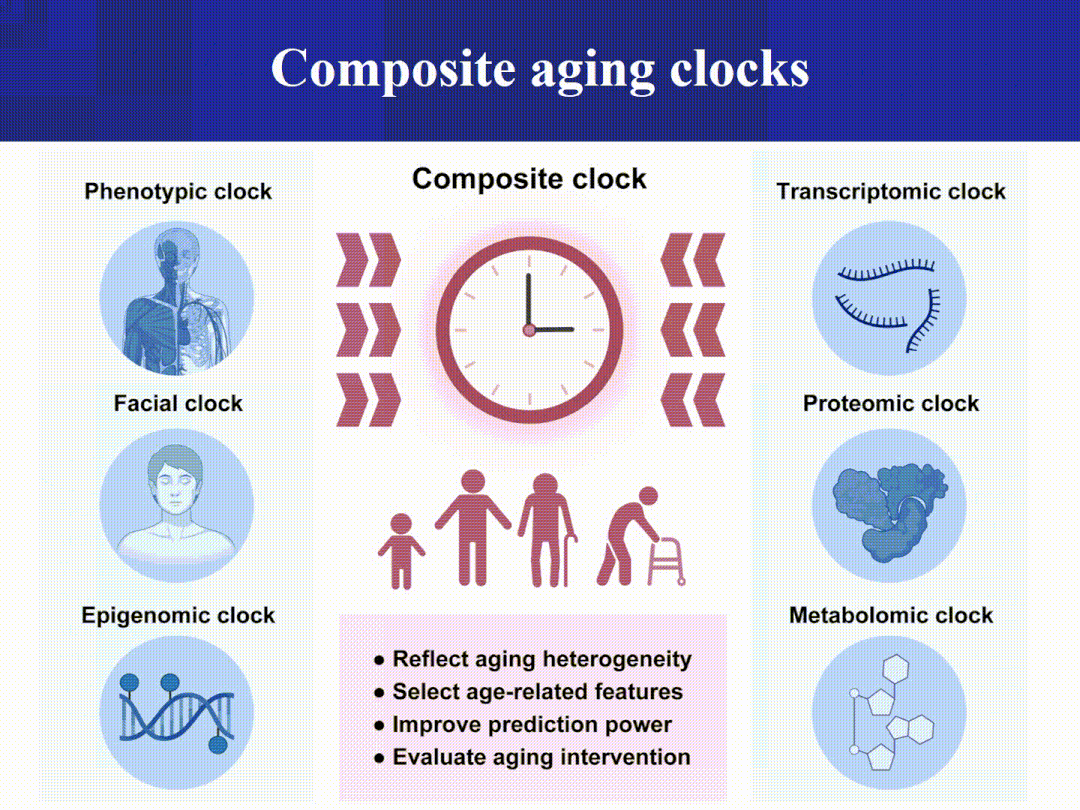
The Aging Clock Matrix:
The DNA methylation clock (Horvath, GrimAge, and their updated versions) infers "biological age" based on methylation patterns at specific CpG sites, enabling it to predict mortality risk, disease risk, and even gauge responses to interventions.
The ATAC/miRNA/transcriptome/proteome/metabolome clock complements the epigenetic clock, jointly profiling system-level damage and responses.
The immune/inflammatory clock and the microbiome clock reflect the interplay between the internal environment and the host.
Imaging and phenotypic clocks (such as facial thermography/3D morphology and retinal photographs) combined with wearable device data offer a minimally invasive, scalable, and continuous monitoring approach. (Nature)
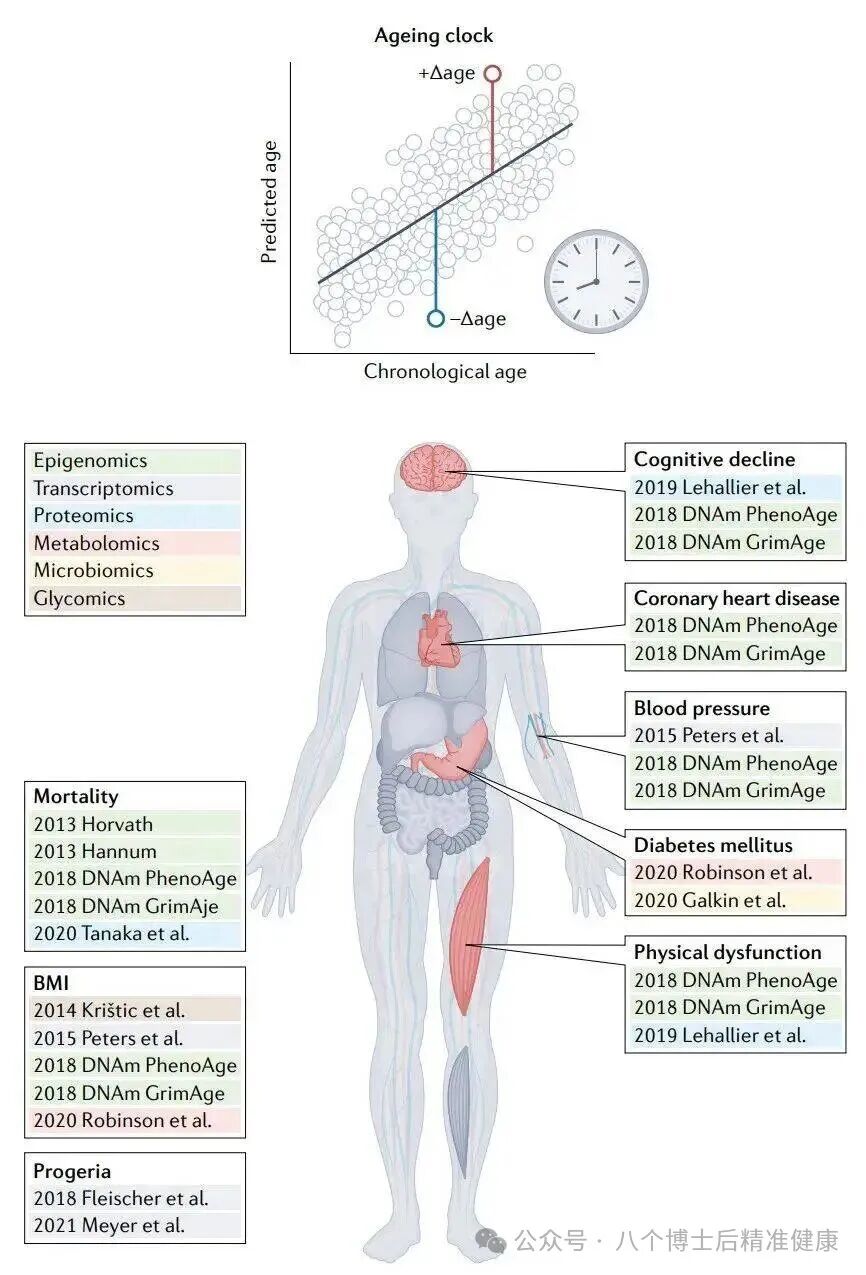
Intervention readout example (supported by primate and human studies):
Metformin: A 40-month study in adult crab-eating macaques reveals that metformin can slow down "epigenetic age" and downregulate aging-related signals such as p21 and IL-1β in multiple tissues. (Science Express)
Taurine: Cross-sectional and supplementation studies suggest that age-related declines in taurine levels are associated with multiple aging phenotypes, while supplementation has been shown to improve bone density, reduce inflammation, and lower oxidative stress markers (8-OHdG) in primate models. (Scientific Association)
The senolytic combination (dasatinib + quercetin) paired with calorie restriction reduces inflammatory and damage markers such as GDF15, MMP9, and IL-6 in aged primates and specific human populations, providing measurable evidence of "molecular-level rejuvenation." (PMC)
General Framework: A cross-review highlighting the Cell and Physiology fields emphasizes that an effective biomarker should meet the "three 'can'-criteria"—reproducibility, interpretability, and responsiveness to interventions. (Cell)
Four NEWS
Cross-Level Coupling: How Signals Are "Amplified Step by Step"—From Cells to Organs to the Individual
Why does the aging of a single cell lead to the aging of an entire organism? The NRMCB review provides the answer: shared biomarkers and transmissible signals. First, ERV/LINE-1 derepression triggers the release of replicative elements and retrovirus-like particles within cells, which spread via paracrine signaling, synchronizing neighboring cells in their transition toward senescence. Second, SASP factors—such as IL-1β, IL-6, TNF, and GDF15—act as "amplifiers" of inflammation and tissue remodeling, creating a localized "pro-senescence microenvironment." Finally, these molecules enter the bloodstream, becoming systemic indicators (detectable in serum or plasma) that drive functional decline across multiple tissues and accelerate phenotypic aging.
In other words, aging is not a discrete event but rather a cascading process triggered at the cellular level, amplified through tissue networks, and ultimately solidified at the organismal level. This is precisely why many biomarkers observed in the brain, cardiovascular system, liver, lungs, kidneys, and immune system can also be "mirrored" at the cellular and blood levels. It also explains why "arterial aging first"—given that blood vessels are the most extensively distributed organs throughout the body—can have far-reaching effects on the entire organism. Moreover, it clarifies why circulating markers such as NfL, GDF15, and CRP exhibit stronger predictive power for the "overall rate of aging"! (Nature)
Key points for practice:
Multi-index combined panels in research and clinical settings outperform single indices;
Cross-level mapping—such as the four-in-one framework of "tissue imaging—interstitial fluid—peripheral blood—functional testing"—represents a practical approach for evaluating the efficacy and side effects of interventions.
Primate models bridge the "last mile" from mechanism to clinic, enabling the assessment of intervention "human relevance" within a reasonable time frame. (PMC)
Five NEWS
Summary
Aging isn’t a “single-molecule curve”—it’s rather a cross-level, cross-organ, and cross-pathway “symphonic score.” In this new NRMCB review, the authors lay out the score clearly: cells exhibit ten distinct fingerprint types, organs have their own unique priorities, and individuals possess universal readouts paired with a clock-like system. These elements mirror one another, amplifying across multiple layers to form a comprehensive framework for aging—one that can be measured, compared, and iteratively refined.

For researchers, it is the unified clock of metrics and methodology; for clinical practice and industry, it serves as the measuring scale that bridges "seeing aging" to "managing aging." The next step involves building a panelized system centered around "standardization, traceability, and responsiveness," complemented by a triple pathway—validation, translation, and regulation—that spans from primates to human populations. Indeed, the tipping point in aging medicine is fast approaching! (Nature)
Recommended News
Share:






